A dual I-O delivering
durable and superior efficacy*
for 1L mMel patients1,2

A dual I-O delivering
durable and superior efficacy*
for 1L mMel patients1,2
Actor portrayal.
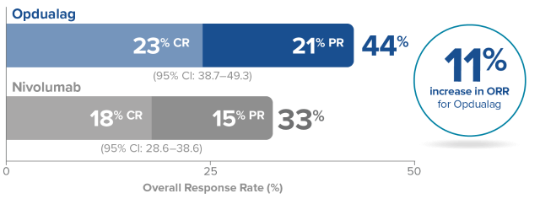
*Based on the 13.2-month median final analysis, the primary endpoint of PFS was statistically significant; mPFS† was 10.1 months (95% CI: 6.4–15.7) with Opdualag vs 4.6 months (95% CI: 3.4–5.6) with nivolumab‡ (HR=0.75§; 95% CI: 0.62–0.92; P=0.0055¶).1,3 ¶At the median follow-up of 19.3 months, the final analysis for the secondary endpoint of OS was not statistically significant (HR=0.80; 95% CI: 0.64–1.01; P=0.0593); threshold for significance was P<0.04302.1,4 ORR could not be formally tested based on testing hierarchy and was descriptively analyzed for Opdualag (43%; 95% CI: 38–48) vs nivolumab (33%; 95% CI: 28–38).1,2,4
†Assessed by BICR.1 ‡Kaplan-Meier estimate.1 §Based on stratified Cox proportional hazards model.1
All data shown below is 4-year data based on minimum potential follow-up of 45.3 months.2
Symbols represent censored observations.
4-year data2:
Primary analysis (19.3-month median follow-up)1,4:
Endpoints were tested in hierarchical order: PFS, OS, ORR.1
†Based on stratified Cox proportional hazards model.1
1L=first-line; BICR=blinded independent central review; CI=confidence interval; HR=hazard ratio; I-O=immuno-oncology; mMel=metastatic melanoma; mPFS=median progression-free survival; NR=not reached; ORR=overall response rate; OS=overall survival; PFS=progression-free survival.
Symbols represent censored observations.
4-year data2:
*Primary analysis (13.2-month median follow-up)1,2:
*Kaplan-Meier estimate.1 †Based on stratified Cox proportional hazards model.1 ‡Based on stratified log-rank test.1

Transcript still to come.
Nivolumab and Relatlimab-rmbw (Opdualag®) is recommended as an NCCN Category 1, preferred first-line treatment option for unresectable or metastatic melanoma, regardless of BRAF status5
*Combination immune checkpoint blockade is associated with improved response rate, PFS, and OS compared with anti-PD-1 monotherapy. Considerations for using combination therapy versus monotherapy include: patient’s desire for potentially improved efficacy and willingness to take on a higher risk of toxicity; absence of comorbidities or autoimmune processes that would elevate the risk of irAEs; tumor burden and patient social support and preparedness to work with medical team to handle toxicities.4 †High-volume symptomatic disease BRAF+ patients may benefit from BRAF/MEK inhibition, as opposed to combination immunotherapy. Otherwise, nivolumab + ipilimumab is preferred first-line over BRAF/MEK therapy due to OS benefit.4 Category 1: Based upon high-level evidence (≥1 randomized phase 3 trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the Panel) that the intervention is appropriate; Preferred intervention: Interventions that are based on superior efficacy, safety, and evidence; and when appropriate, affordability; Other recommended intervention: Other interventions that may be somewhat less efficacious, more toxic, or based on less mature data; or significantly less affordable for similar outcomes.5
BRAF=B-raf proto oncogene; irAE=immune-related adverse events; MEK=mitogen-activated protein kinase; NCCN=National Comprehensive Cancer Network® (NCCN®); PD-1=programmed cell death protein-1.
Symbols represent censored observations. Based on an exploratory analysis.
BRAF mutant PFS 4-year data6:
BRAF wild-type PFS 4-year data6:
*Based on unstratified Cox proportional hazards model.1
mDOR was not yet reached for Opdualag at 4 years
Opdualag mDOR: NR (95% CI, months: 47.7–NR); nivolumab mDOR: 56.0 (95% CI, months: 43.2–NR)2
Primary analysis (19.3-month median follow-up)1,2:
4-year data based on minimum potential follow-up of 45.3 months.2
*At the time of the final OS analysis, which was event-driven and occurred after the final PFS analysis.1 †Not formally tested based on the testing hierarchy.1
CR=complete response; mDOR=median duration of response; PR=partial response.
Find information about adverse reactions seen in the clinical trial.
Learn more about the dosing schedule for this fixed-dose combination therapy.
References: